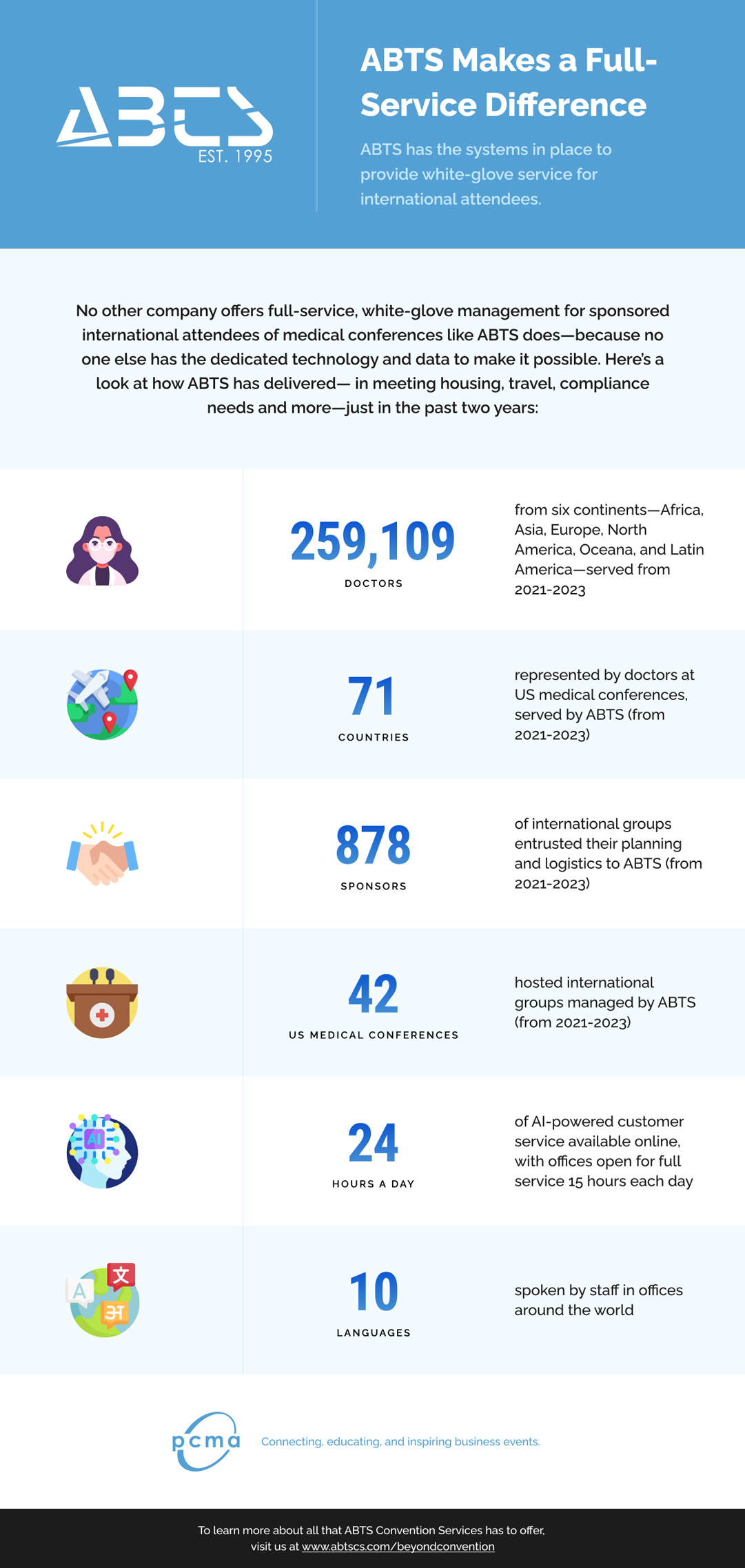
ABTS is the only provider of white glove travel, lodging, concierge, and compliance services for international attendees of major medical association events.
ABTS Convention Services is the only provider of white glove travel, lodging, concierge, and compliance services for international attendees of major medical association events. And thanks to state-of-the-art technology and data analysis, their numbers of satisfied attendees and sponsors are growing around the world.